Expanded Eligibility for COVID-19 Vaccine Booster Doses for Children 5-11 Years Old
More than 4.8 million children ages 5 through 11 have been diagnosed with COVID-19, and 15,000 have been hospitalized since the beginning of the pandemic, according to the Centers for Disease Control and Prevention (CDC). As numbers of cases fluctuate throughout the country, a booster dose will be useful to safely help enhance protections against severe disease.
On May 19, 2022, the U.S. Food and Drug Administration (FDA) amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to children 5 through 11 years old at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. Additionally, the CDC both agreed to this expansion to booster authorizations and recommended that those 12 years of age and older who are immunocompromised and those 50 years of age and older should receive a second booster dose at least 4 months after their first.
A few days after the recommendations were made by the FDA and CDC, the NYC Department of Health and Mental Hygiene announced that the Pfizer COVID-19 vaccine boosters for children ages 5 to 11 have become widely available in New York City. Boosters are available at various sites including pharmacies, community health centers, hospitals, and City-run clinics that provide free COVID-19 vaccines.
As of June 3, 2022, there have been over 3 million additional or booster doses administered to people of all ages in NYC. Over 100,000 children ages 5 through 17 have had an additional dose.
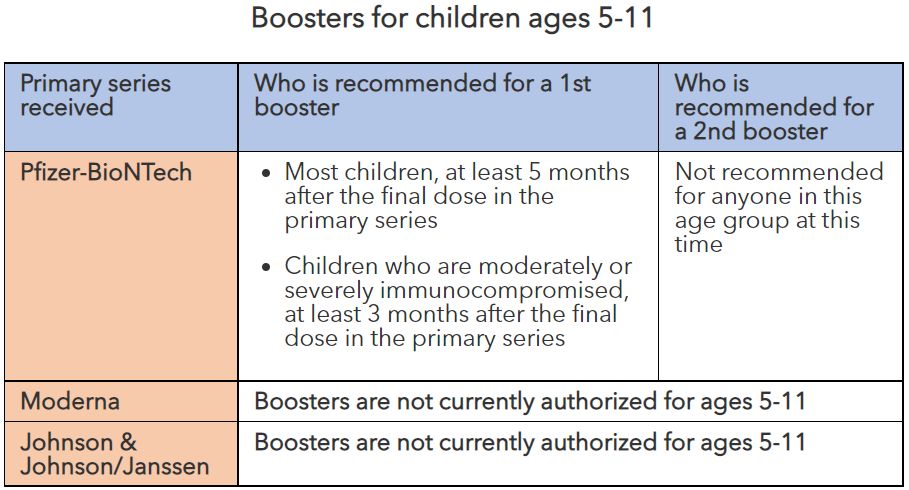
Boosters for children ages 5-11
| Primary series received | Who is recommended for a 1st booster | Who is recommended for a 2nd booster |
|---|---|---|
| Pfizer-BioNTech |
|
Not recommended for anyone in this age group at this time |
| Moderna | Boosters are not currently authorized for ages 5-11 | |
| Johnson & Johnson/Janssen | Boosters are not currently authorized for ages 5-11 | |
Vaccines and boosters remain the best tools to combat the severity of disease and hospitalizations in all age groups.
Visit the NYC COVID-19 and Flu Finder to find a vaccination location near you and get booster vaccinations for anyone in your household who is between 5 to 11 years old today.
Stay informed
NYC Department of Health and Mental Hygiene
New York State Department of Health
Centers for Disease Control and Prevention
COVID-19 Vaccines for Moderately or Severely Immunocompromised People
U.S. Food and Drug Administration